Describe Hydrogen Bonding Between Water Molecules
In H 2 O only two of the six outer-shell electrons of oxygen are used for this purpose leaving four electrons which are organized into two non-bonding pairs. A hydrogen bond in water occurs between the hydrogen atom of one water molecule and the lone pair of electrons on an oxygen atom of a neighboring water molecule.
Hydrogen Bond Definition Properties Types Formation Examples
This happens with all water molecules leaving the oxygen with more electrons and ultimately the more negative side.

. In water each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them. The strong dipole of water exerts electrostatic and gravitational forces on charged electrovalent compounds and on the dipoles of polar covalent compounds. Lower alcohols are soluble in water because of the hydrogen bonding which can take place between water and.
Which of the following statements describe hydrogen bonding between water molecules or describe properties of water that lead to hydrogen bonding between water molecules1The slightly negative oxygen atom of one water molecule is attracted to the slightly positive hydrogen atom of another water molecule2The slightly positive hydrogen atom is. Viscosity and surface. This is because there is a big difference in electronegativity between the oxygen and the water.
But the intermolecular bonds the bonds BETWEEN water molecules are the result of hydrogen bonding. 2The slightly positive hydrogen atom is attracted to the slightly negative oxygen atom within a single water molecule. And hydrogen bonding occurs when hydrogen is bound to a strongly electronegative element such as oxygen or nitrogen or fluorine.
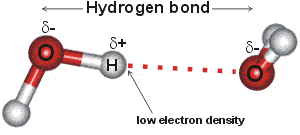
Hydrogen bonds between water molecules Hydrogen bonds form when a slightly negatively charged part of a molecule comes close to a slightly positively charged hydrogen molecule in the same or another molecule. Such hydrogen-bonding interaction is VERY STRONG INTERMOLECULAR. Describe the structure and geometry of a water molecule and explain what properties emerge as a result of this structure.
The slightly negative oxygen atom of one water molecule is attracted to the slightly positive hydrogen atom of another water molecule. There is a weak intermolecular force of attraction between the patially positively charged hydrogen atom of one molecule and the. Properties of Hydrogen Bonding Solubility.
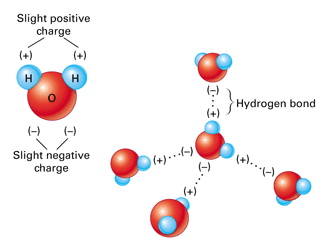
The image above depicts water molecules. And positive and negative forces attract so the now positive hydrogens will bond with the now negative oxygens. Hydrogen Bonding occurs between 2 adjacent molecules of H 2 O.
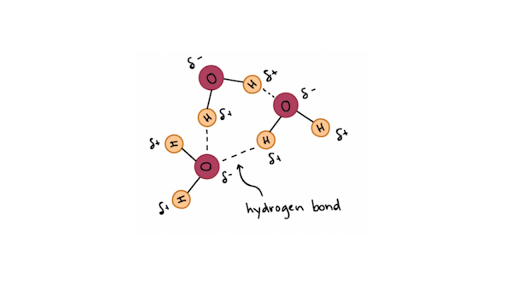
Ø The bond dissociation energy of covalent bond between O and H in water molecule is 470 kJmol. Hydrogen bonds form between neighboring water molecules when the hydrogen of one atom comes between the oxygen atoms of its own molecule and that of its neighbor. H δ O δ H δ O δ H 2δ O δ H 2.
It is known that oxygen is very electronegative therefore it attracts the electrons creating a polar bond. The adhesion of water is explained by hydrogen bonding of water molecules to other polar surfaces. A The slightly positive hydrogen atom is attracted to the slightly negative oxygen atom within a single water molecule.
This happens because the hydrogen atom is attracted to both its own oxygen and other oxygen atoms that come close enough. - The bond between oxygen and hydrogen in water H2O - Electrons are not equally shared by the atoms in a covalent bond - One atom of a covalent bond has higher electronegativity than the other atom in the bond Nonpolar bonds. In the case of water hydrogen bonds form between neighboring hydrogen and oxygen atoms of adjacent water molecules.
1 Water the molecule. Water is a polar molecule. Which of the statements describe hydrogen bonding between water molecules or describe properties of water that lead to hydrogen bonding between water molecules.
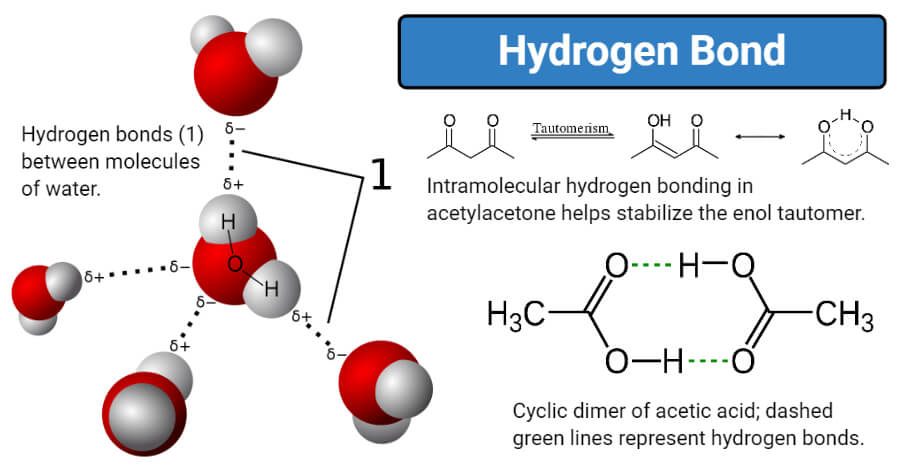
Ø Hydrogen bond formed between two molecules is called intramolecular hydrogen bond. Water molecules have a molecular formula of H2O. The polar covalent bond between oxygen and hydrogen creates negative and positive partial charges respectively.
Hydrogen bonding is an attractive force between two molecules that relies on the slight polarity of the O-H O-F or O-N bond. As the compounds involving hydrogen bonding between different molecules have a higher boiling point so they. They are often described as interactions.
Explain why hydrogen bonding occurs between water molecules. If youre behind a web filter please make sure that the. Objectives Describe how water contributes to the fitness of the environment to support life.
This is most easily seen in water Hydrogen bonds are not strong bonds. The attraction between individual water molecules creates a bond known as a hydrogen bond. If youre seeing this message it means were having trouble loading external resources on our website.
Ø The bond dissociation energy of hydrogen bond in liquid water is about 23 kJmol. B A water molecule is polar because it has a bent structure and its shared electrons. Ø Hydrogen bonds in water are very unstable and they frequently break and.
- The bond between hydrogen atoms in diatomic hydrogen H2 - Electrons are equally shared between atoms in a covalent bond. 3Water is polar due to its bent structure and an unequal sharing of electrons. Most importantly it bonds to other water molecules using a special kind of interaction called a hydrogen bond which is a weak intermolecular bond between a positively charged hydrogen atom and a negatively charged atom.
1The slightly negative oxygen atom of one water molecule is attracted to the slightly positive hydrogen atom of another water molecule. Explain the relationship between the polar nature of water and its ability to form hydrogen bonds. They form bonds using polarity and use a very strong dipole dipole effect.
A hydrogen bond is an intermolecular attractive force in which a hydrogen atom that is covalently bonded to a small highly electronegative atom is attracted to a lone pair of electrons on an atom in a neighboring. A hydrogen bond is a partially electrostatic attraction between a hydrogen H atom which is bound to a more electronegative atom or group such as nitrogen N oxygen O or fluorine Fthe hydrogen bond donorand another adjacent atom bearing a lone pair of electronsthe hydrogen bond acceptor. List five characteristics of water that are emergent properties.
Model of hydrogen bonds 1 between molecules of water. As a polar molecule with oppositely charged ends water is able to readily form bonds with other polar or charged molecules or atoms. Explain how hydrogen bonding occurs between molecules of water.
Due to the electronegativity difference between the atom pairs mentioned electrons are unevenly shared across the covalent bond. The structure of water molecules and how they can interact to form hydrogen bonds. The O-H bond in water is a polar bond.
Hydrogen bonds are attractions of electrostatic force caused by the difference in charge between slightly positive hydrogen ions and other slightly negative ions. A water molecule is polar because it has a bent structure and its shared electrons are more attracted to. Molecules Biodiversity Food and Health ALM June 2010 Biological Molecules Biological Molecules a describe how hydrogen bonding occurs between water molecules and relate this and other properties of water to the roles of water in living organisms.

Hydrogen Bond Definition Types And Examples
Hydrogen Bonds In Water Article Khan Academy
Answer In General Chemistry For Jude 101935

Hydrogen Bonding Chemistry For Non Majors

Hydrogen Bonding Chemistry For Non Majors

Chemistry Tutorial Hydrogen Bond Water Molecule Molecules

File 210 Hydrogen Bonds Between Water Molecules 01 Jpg Wikimedia Commons
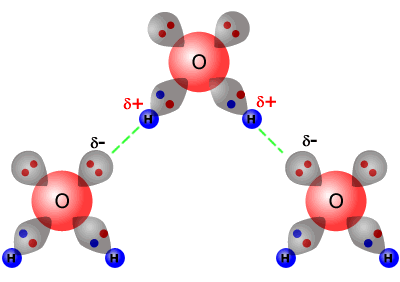
Structure And Bonding 2 43 Hydrogen Bonding

Hydrogen Bonds In Water Article Khan Academy
Schematics Of The Atomic Structure Of Water Molecule And The Hydrogen Download Scientific Diagram

Hydrogen Bond Ck 12 Foundation

The Strong Polar Bond Between Water Molecules Creates Water Cohesion U S Geological Survey

3 10 Water Is The Highest Cohesive Of The Non Metallic Liquids Cohesion Embraces Hydrogen Bonds Together To Chemistry Chemistry Classroom Teaching Chemistry

Density Miscibility Lesson Hydrogen Bond Chemistry Lessons Water Molecule